Curing HIV Disease With Stem Cell Therapy
Science Highlights by Ann A. Kiessling, PhD
What is HIV disease?
Human Immunodeficiency Virus (HIV) infects specific types of cells in the immune system. Like most viruses, in order for HIV to infect a cell, the virus must bind to a specific protein, termed a receptor, on the cell’s surface. There are many different types of cells in our immune system, and each plays a specific role in fighting infections, both bacterial and viral. Our bodies produce billions of new immune cells every day from stem cell reservoirs in bone marrow.
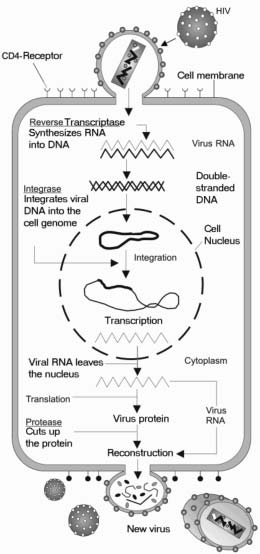
HIV has a complex life cycle that includes becoming part of the genetic information of the host cell so the cell is infected for life. Infection can be dormant, with no new virus produced, or active, with new virus produced continuously
HIV infects immune cells that have a protein termed CD4 on their surface. Some HIV-infected CD4 cells die, but others remain in the body, prepared to fight another infection at a later date. When the HIV infected person encounters a new infection, such as the flu, or an infected injury, the HIV-infected CD4 cell responds like a reliable member of the immune system. It becomes activated, multiplies, and as a side effect, produces new HIV particles before it dies. The new HIV particles then infect new CD4 cells, setting up a repeat of the cycle. Because billions of new immune cells are made every day, it generally takes several years for an HIV infected individual to lose enough CD4 cells to have a negative impact on his/her ability to fight other infections. Once the number of CD4 cells is depleted to the point that the individual can no longer effectively fight new infections, their HIV disease has advanced to a new condition termed Acquired Immunodeficiency Syndrome (AIDS).
Virus receptor: the protein on the surface of a cell that allows the virus to bind to, and then enter, the cell to infect it
Is there a cure for HIV infection?
No. It is currently treated with drugs that block specific steps in the life cycle of HIV infection in the CD4 cells, but because some CD4 cells live for decades, and are not killed by the HIV drugs, the potential for them to activate, multiply, and give rise to new virus particles persists for decades. The long life of immune cells is important for disease memory, i.e. it is the reason adults don’t get childhood diseases, such as chicken pox, and the reason that vaccination is effective against diseases, such as polio, for many decades.
Immune system: the collection of cells that respond to and eliminate infection and foreign cell invaders
Can stem cells cure HIV disease?
Over 50 years ago, treatments for some diseases of the immune system were developed, and are the original stem cell therapies. The treatments involve destroying all the diseased immune cells, such as leukemias, with radiation treatment and cancer drugs. (6,7,8). Once the diseased immune system is destroyed, it is replaced by transplanting new immune cells from the bone marrow of a healthy donor.
Bone marrow transplant: the transfer of healthy bone marrow stem cells from a donor to a recipient whose own immune system has been destroyed
This has now become a routine treatment for many cancers and diseases of the blood(1). Early in the HIV pandemic, it was recognized that bone marrow transplants might cure HIV disease. But obstacles have stood in the way of this therapeutic approach:
First, all of the HIV-infected CD4 cells in the recipient must be destroyed before the transplant. If not, the donor bone marrow cells will become infected with HIV, and the transplant will have been for naught. Since not all CD4 cells everywhere in the body are destroyed by the radiation and drugs, infection of transplanted bone marrow was observed (2). Since bone marrow is limited in supply, the medical community was reluctant to “waste” valuable bone marrow to infection by HIV.
Second, the transplanted bone marrow must be a perfect match to the recipient’s cells, or the new immune system will attack them as “foreign,” leading to a life threatening condition known as “graft versus host disease” (see: Patient Specific Stem Cells). Since few matches are perfect, bone marrow recipients are usually treated with immune suppressing drugs. Since immune suppression of HIV infected persons leads to AIDS, this possibility further limited enthusiasm for bone marrow transplant treatment for HIV disease, and restricted it to those individuals who also developed a cancer for which bone marrow transplant was needed.
Importantly, proof-of-concept for the efficacy of bone marrow transplant for HIV disease was reported in 2009 in the New England Journal of Medicine(3). A team of German physicians treating an HIV-infected man with a cancer, lymphoma, by bone marrow transplant, was able to use a bone marrow match from an individual who was naturally resistant to HIV infection. Unlike earlier reports, the new bone marrow cells did not become infected with HIV.
What is natural resistance to HIV infection?
Studies of persons routinely exposed to HIV, but who did not become infected, revealed that in addition to cells having the CD4 protein, efficient infection also needs one of two additional receptor proteins, termed CXCR4 and CCR5. CXCR4 is a protein expressed on the surface of many cells, not just CD4 cells, but CCR5 is less commonly expressed. Individuals genetically lacking CCR5 appear normal and demonstrate remarkable resistance to HIV infection. The bone marrow donor for the German patient was genetically lacking the CCR5 protein.
How can stem cells provide therapy for HIV disease?
The proof-of-concept report from Germany supports the value of bone marrow transplant for HIV disease. New developments in stem cell science open new avenues to solve the main barriers to this therapeutic approach.
First, the possibility of deriving patient-specific stem cells (see: Patient-specific stem cells) will eliminate wasting valuable bone marrow.
Second, the laboratory methods for developing bone marrow stem cells from patient-specific stem cells have greatly advanced in the past two years (4), thus eliminating the need for a good tissue match from a bone marrow bank.
Third, the laboratory methods for silencing genes in stem cells has also greatly advanced in the past two years(5).
Taken together, it is now possible to derive patient-specific stem cells from HIV-infected individuals, differentiate them into bone marrow stem cells, and knock-out the CCR5 protein, rendering them resistant to HIV infection. This source of cells would then be available for transplant into the HIV infected individual, who may or may not have to prepare by going through radiation and drug treatment for complete ablation of all HIV-infected cells. Because the new cells will not be susceptible to HIV infection, it may be possible that over time, they would simply replace the individual’s HIV infected cells.
What is the timeline to develop patient-specific, CCR5 negative, bone marrow stem cells for HIV treatment?
The science of patient-specific stem cells is moving rapidly. By mid-2011, the best sources could be at hand. Within the same time frame, the most efficient laboratory methods for developing stem cells into bone marrow stem cells will also be identified. Hence, 2012 is a realistic time frame for the development of reliable methods to derive patient-specific bone marrow stem cells.
Laboratory methods to knock-out the CCR5 protein may also take 2 to 3 years. Several approaches are currently under study(5).
Once the CCR5 negative, patient-specific bone marrow stem cells are at hand, possibly by 2013, they must be studied for safety and efficacy. This may be the longest phase of the work since it will be necessary to prove long-term survival and lack of negative side effects in an animal model. A conservative estimate for this phase is 3 to 5 years.
Hence, if funding is available, it will be known within 5 to 8 years if patient-specific, CCR5-negative, bone marrow stem cells are a useful tool in the fight against HIV disease.
Will the cost be too high?
Until the efficiencies with which patient-specific, CCR5-negative, bone marrow stem cells can be derived are known, it will not be possible to predict overall costs per treatment.
However, given the current cost of $25,000 to $50,000 per year per patient for monitoring and treating HIV disease in the U.S., it is highly likely that stem cell therapy may be substantially less costly.
Bedford Research scientists will begin the patient-specific Testis Stem Cell Project in 2010, as soon as funding is available.
References:
- Kiessling AA and Anderson SC 2007 Human Embryonic Stem Cells, Jones and Bartlett plublishers
- Krishnan A,Zaia J, and Forman SJ 2003. Should HIV-positive patients with lymphoma be offered stem cell transplants? Bone Marrow Transplantation 32: 741-748
- Hutter G, Nowak D, Mosner M, Ganepola S, Mubig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau I, Hofmann W, Thiel E 2009. New England Journal of Medicine 360: 693-698.
- Goodrich A, Ersek A, Varain N, Groza D, Cenariu M, Thain D, Almeida-Porada G, Porada C, Zanjani E 2010. In vivo generation of b-cell-like cells from CD34+ cells differentiated from human embryonic stem cells. Experimental Hematology 38: 516-525.
- Shimizu S, Hong P, Arumugam B, Pokomo Ll, Boyer J, Koizumi N, Kittipongdaja P, Chen A, Bristol G, Ballic Z, Zack J, Yang O, Chen I, Lee B, An D 2010. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 115: 1534-1544.
