Frequently Asked Questions
Welcome to one of our most popular resources. These simple and clear answers to Frequently Asked Questions are intended to give you a basic understanding of stem cells and stem cell research.
Stem Cells
- What is a stem cell?
- How many kinds of stem cells are there?
- What are adult stem cells?
- Do all adult tissues and organs have stem cells?
- What are fetal stem cells?
- Can fetal stem cells repair adult organs and tissues?
- What are cord blood stem cells?
- What is a clone?
Pluripotent Stem Cells
- Types of pluripotent stem cells
- What are embryonic stem cells? – video!
- What are parthenote stem cells? – video!
- What is an induced pluripotent stem cell? – video!
- What are nuclear transplant stem cells? – Video!
- What are other sources of pluripotent stem cells?
- What is the best type of stem cell to use for therapy?
What is a stem cell?
A reserve cell with the capacity to grow and multiply to replace dead or damaged adult cells. Some, but not all, organs and tissues in the body have a supply of stem cells – skin is an example: skin wounds are repaired by skin stem cells, similarly, liver damage is repaired by liver stem cells. Reserve stem cells do not, however, exist for many vital tissues, including: heart, spinal cord, brain and pancreas. Scientists are developing new sources of stem cells for these tissues.
Key Term: “Pluripotent” (adjective): The capacity to become any cell in the body.
- Pluripotent stem cells show the most promise for use in stem cell therapies.
- Embryonic stem cells are pluripotent, adult stem cells are not.
How many kinds of stem cells are there?
The answer to this question is not known with certainty. Researchers in the field divide stem cells into four broad categories:
- adult stem cells,
- fetal stem cells,
- embryonic stem cells, and
- more recently(2007), induced stem cells.
These categories refer to the source of the cells, but do not describe their nature.
A new type of stem cell, nuclear transplant stem cells (“ovasomes”), takes advantage of new methods to create stem cells. Some of the methods are the same as those used for cloning animals, and thus, unfortunately, this new methodology has been highly controversial.
Another new type of stem cell, parthenote stem cells from “activated eggs”, takes advantage of new methods to stimulate unfertilized eggs to divide. This new category has received less public debate because it is less common and less well understood. It is becoming clear, however, that parthenote stem cells may have important therapeutic potential.
Types of Pluripotent Stem Cells
1) Embryonic stem cells from fertilized eggs are good models for research, but they have ethical issues, and will have tissue rejection problems (similar to bone marrow and kidney transplants).
2) Parthenote stem cells (derived from unfertilized eggs, “activated eggs”) may be as pluripotent as embryonic stem cells, and have been the focus of BRF scientists for several years. Studies using monkey parthenote stem cells to treat Parkinson’s disease have been very promising.
- Parthenotes do not have the potential tissue rejection problems faced by stem cells derived from fertilized eggs.
- Unlike adult stem cells, parthenotes can potentially become any cell in the body.
Less controversial than stem cells - that are derived from fertilized eggs.
3) Induced Pluripotent stem cells (derived by adding proteins that reprogram adults cells, reverting them to their embryonic state) “These new cells are expected to live for a very long time while retaining the ability to form all of the different tissues found in a human body.” Ian Wilmut, Time, April ’08
What are adult stem cells?
Adult stem cells are the reserve supply of cells that can multiply when needed for repair of adult organs and tissues. Skin is an example: skin wounds are repaired by skin stem cells, similarly, liver damage is repaired by liver stem cells.
Adult stem cells start out as embryonic stem cells, then become fetal stem cells, and mature into adult stem cells. For example, if it takes 20 maturation steps for an embryonic stem cell to turn into a mature skin cell, skin stem cells are at step 15; they are not quite mature skin cells, but they cannot back-up to become another cell type, such as a heart muscle cell.
Do all adult tissues and organs have stem cells?
This is not known for certain and is an active area of research. It has long been thought that nerves, such as the spinal cord and brain, heart and kidney are examples of organs that do not contain a reservoir of stem cells. It is possible, however, that these organs and tissues do have a small reservoir of stem cells that may be encouraged to multiply if the right conditions were known. Whether or not sufficient numbers can be produced to cure such problems as Parkinson’s Disease and Heart Failure is not known.
What are fetal stem cells?
The developing organs and tissues in a fetus contain a relatively large supply of stem cells because they are needed for growth and maturation. The difference between embryonic stem cells and fetal stem cells is the fetal stem cells have matured part of the way to mature cells. For example, if it takes 20 maturation steps for an embryonic stem cell to turn into a mature skin cell, fetal skin cells are at step 10; they are not as mature as adult skin stem cells, but they are past the stage of becoming committed to the liver.
Can fetal stem cells repair adult organs and tissues?
Possibly. There are currently several problems with the therapeutic use of fetal stem cells.
First, fetal tissue research is highly controversial. There are significant moral and ethical issues with the use of fetal tissues for research purposes. Second, the numbers of stem cells in fetal tissues may not be sufficient for the therapeutic needs of adults. Thus, methods need to be developed to greatly expand the supply of fetal stem cells if they are to be therapeutically useful. Third, tissue rejection problems similar to those encountered in kidney and heart transplants may limit the usefulness of fetal stem cells.
What are cord blood stem cells?
Cells in the umbilical cord are “multipotent” and can give rise to all the cells in normal bone marrow. Scientists are working to discover if cord blood stem cells can multiply and become other types of adult stem cells. For this reason many new parents have their new baby’s umbilical cord blood cryopreserved for potential future use.
What are embryonic stem cells?
Eggs fertilized by sperm begin to divide into multiple cells, but do not begin to form organs and tissues for at least two weeks. During this early developmental period, the cells that will ultimately give rise to the developing fetus can be encouraged to grow indefinitely in the laboratory as stem cells that are not committed to any particular tissue. With the right mixture of hormones and growth factors, such laboratory-grown embryonic stem cells can be encouraged to become many types of adult cells such as: nerves, heart muscle, and insulin-producing cells. Animal models, such as mice, have been used to demonstrate the important therapeutic potential of these cells in treating the symptoms of such diseases as Parkinson’s Disease and Diabetes.
There are two main problems with the use of embryonic stem cells for therapeutic purposes. First is the moral and ethical debate that surrounds the use of a fertilized human egg for research and therapy. Second is the potential for tissue rejection (similar to the rejection in a liver or blood transplant) that could limit the therapeutic usefulness of embryonic stem cells. For this reason the Bedford Research Foundation is focused on developing stem cells from unfertilized eggs.
An egg ready to be fertilized extrudes one half of its chromosomes (top left) to make room for the sperm’s chromosomes. The sperm enters the egg, activates it, and the egg begins to divide into smaller (bottom left) and smaller cells (top right, a “morula”). At approximately 100 cells, the cells on the outside form a sealed layer that surrounds a fluid-filled cavity with a group of cells inside (Blastocyst). The inside cells (small red) can be removed to a petri dish (bottom right) and will continue to divide into embryonic stem cells.
What are parthenote stem cells?
It has been known for many years that human eggs occasionally undergo spontaneous cell divisions. These dividing eggs lead to dermoid cysts and to benign tumors known as teratomas, that contain several cell types including skin and hair. If eggs could be routinely stimulated to undergo cell division in the laboratory, this could be an especially valuable source of stem cells that could bypass the moral, ethical, and some of the tissue rejection problems associated with fetal and embryonic stem cells, particularly for the woman donating the egg. For example, a woman with diabetes or spinal cord injury, could donate her own eggs for her own stem cells.
Update – December 19, 2007: International Stem Cell Corporation
International Stem Cell Corporation recently announced that scientific team, led by Chief Scientist, Dr. Elena Revazova, has created a new class of human stem cell lines that do not involve the use of fertilized eggs and may enable hundreds of millions of people of different sex, ages and racial groups to benefit from cell based therapy with cells that will not be rejected by the patients own immune system after transplanting. The article was published in the on line edition of the well known peer-review publication, Cloning and Stem Cells on December 19, 2007
Schematic of Parthenote stem cells.
An egg ready to be fertilized (top left) is activated by chemicals or a small electrical jolt. The activated egg divides into smaller (bottom left) and smaller (top right, a morula) cells. At approximately 100 cells, the cells on the outside form a sealed layer that surrounds a fluid-filled cavity with a group of cells inside (Blastocyst). The inside cells (small red) can be removed to a petri dish (bottom right) and will continue to divide into parthenote stem cells.
What is an induced pluripotent stem cell?
Discovered by Shinya Yamanaka, MD, PhD at Japan’s Kyoto University in 2007, these new stem cells give rise to a totally new category of pluripotent stem cell. “Yamanaka screened 24 candidate proteins before finding four that were able to reprogram adult cells, reverting them to their embryonic state. He and others then showed that these factors are also effective in human cells. Developmental biologist James Thomson, of the University of Wisconsin was the first to identify a slightly different group of factors that do the same.” – Ian Wilmut, Time, April ’08 More References About Induced Pluripotent Stem Cells
 Original Publication from Cell: Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors (pdf)
Original Publication from Cell: Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors (pdf)
Kazutoshi Takahashi, Koji Tanabe, Mari Ohnuki, Megumi Narita, Tomoko Ichisaka, Kiichiro Tomoda, and Shinya Yamanaka
DOI 10.1016/j.cell.2007.11.019
Excerpt: “SUMMARY: Successful reprogramming of differentiated human somatic cells into a pluripotent state would allow creation of patient- and disease-specific stem cells. We previously reported generation of induced pluripotent stem (iPS) cells, capable of germline transmission, from mouse somatic cells by transduction of four defined transcription factors. Here, we demonstrate the generation of iPS cells from adult human dermal fibroblasts with the same four factors: Oct3/4, Sox2, Klf4, and c-Myc. Human iPS cells were similar to human embryonic stem (ES) cells in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity. Furthermore, these cells could differentiate into cell types of the three germ layers in vitro and in teratomas. These findings demonstrate that iPS cells can be generated from adult human fibroblasts.”
 Wikipedia: Induced pluripotent stem cell
Wikipedia: Induced pluripotent stem cell
Excerpt: “Induced pluripotent stem cells were first generated by Shinya Yamanaka’s team at Kyoto University, Japan in 2006. Yamanaka had identified genes that are particularly active in embryonic stem cells, and used retroviruses to transfect mouse fibroblasts with a selection of those genes. Eventually, four key pluripotency genes essential for the production of pluripotent stem cells were isolated; Oct-3/4, SOX2, c-Myc, and Klf4. Cells were isolated by antibiotic selection for Fbx15+ cells. However, this iPS line showed DNA methylation errors compared to original patterns in ESC lines and failed to produce viable chimeras if injected into developing embryos.”
Video: “Generating iPS Cells from MEFS through Forced Expression of Sox-2, Oct-4, c-Myc, and Klf4”
– published via JOVE, Journal of Visualized Experiments
G. Grant Welstead, Tobias Brambrink, Rudolf Jaenisch, Whitehead Institute for Biomedical Research, Massachusetts Institute of Technology
Article: A Breakthrough on Stem Cells
published in Time, by Alice Nov 20, 2007
Excerpt: “In the journal Cell, Shinya Yamanaka of Kyoto University reports success in turning back the clock on cheek cells from a middle-aged woman, while James Thomson of University of Wisconsin, the first to isolate human embryonic stem cells, achieved the same feat with foreskin cells from a newborn baby.”
Article: Stem cells: The magic brew
published in Nature 448, 260-262 (19 July 2007) | doi:10.1038/448260a; Published online 18 July 2007
by Janet Rossant
Excerpt: “Researchers have engineered embryonic stem-like cells from normal mouse skin cells. If this method can be translated to humans, patient-specific stem cells could be made without the use of donated eggs or embryos.”
Press Release: Reprogrammed adult cells treat sickle-cell anemia in mice
CAMBRIDGE, Mass. (December 6, 2007), Whitehead Institute for Biomedical Research at MIT
Excerpt: “Mice with a human sickle-cell anemia disease trait have been treated successfully in a process that begins by directly reprogramming their own cells to an embryonic-stem-cell-like state, without the use of eggs. This is the first proof-of-principle of therapeutic application in mice of directly reprogrammed “induced pluripotent stem” (IPS) cells, which recently have been derived in mice as well as humans.”
What are nuclear transplant stem cells (ovasomes)?
In the course of trying to understand how cells became committed to each tissue and organ in the body, scientists discovered that if the chromosomes were removed from an egg, and replaced with the chromosomes (in the nucleus) of an adult cell, it was possible to stimulate the egg to begin to divide into multiple cells just as if it had been fertilized with sperm. Thus, nuclear transplantation refers to the process of replacing egg chromosomes with the chromosomes of another cell, usually a cell that has been growing in the laboratory. This research success made it possible to try to clone adult animals. It is important to note that the success rate in cloning animals is very low, fewer than 1% of eggs that undergo nuclear transplantation, but the success rate in stimulating the transplanted egg into dividing into multiple cells is high. Thus, such transplanted eggs may be ideal candidates for stem cells, but not for producing clones.
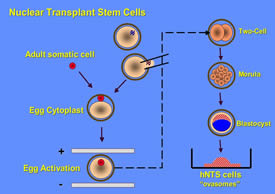 Schematic of the development of nuclear transplant stem cells.
Schematic of the development of nuclear transplant stem cells.
An egg ready to be fertilized (top middle) is stabilized on a microscope state and its chromosomes removed with a fine glass needle. The chromosomes in an adult somatic cell (upper left) are transferred into the egg, and the egg is then activated, by chemicals or a small electrical jolt. The activated, nuclear transplant egg divides into smaller and smaller cells, then forms a blastocyst that looks very much like the blastocyst from a fertilized egg. The inside cells (small red) can be removed to a petri dish (bottom right) and will continue to divide into nuclear transplant stem cells (ovasomes). (Click on image to enlarge.)
What is a clone?
The term clone stems from the Greek word, klon, which means twig. Since twigs can sometimes give rise to new trees, clone has come to mean an adult individual that is genetically identical to another individual. Scientists use the word clone to describe many routine laboratory methods of generating multiple identical copies of something, including bacteria, pieces of DNA, and cultured cells used for research. Identical twins are naturally occurring clones of each other. A laboratory method for producing cloned animals involves transplanting the nucleus of an adult cell into an egg whose chromosomes have been removed. After artificially stimulating the egg with the new nucleus into dividing into several cells, it is placed in the uterus of an animal that thinks she is pregnant. In a small percentage of cases, the “nuclear transplant embryo” can give rise to a pregnancy. Unfortunately, for reasons not understood, such cloned animals have a high incidence of serious health problems.
What are other sources of pluripotent stem cells?
The Foundation is focused on deriving stem cells from unfertilized eggs:
1) Those derived from eggs artificially activated without sperm – “parthenotes”
2) Those derived from eggs whose chromosomes have been removed and replaced by chromosomes from another cell – “nuclear transplant stem cells”
What is the best type of stem cell to use for therapy?
We do not know yet.
Embryonic stem cells are good models for research, but will have tissue rejection problems (similar to bone marrow and kidney transplants) if used for therapies.
Parthenote stem cells (derived from unfertilized eggs) may avoid the tissue rejection problems of embryonic stem cells, but these cells are not currently available for humans. Studies using monkey parthenote stem cells to treat Parkinson’s disease have been promising.
Nuclear transplant stem cells (also derived from unfertilized eggs) may be the best for therapy, but no line of human transplant stem cells now exists.
Induced pluripotent stem cells (derived by “reprogramming” protein in adult cells) “One day iPS cells may be used to replace cells damaged or lost in disease, but much remains to be learned before such therapy would be appropriate. As a step along the way, iPS cells from patients with an inherited disease will offer opportunities to study illnesses such as als and Parkinson’s and psychological ailments, as scientists program the cells back to their embryonic state and watch them mature in the lab. In the process, they may pinpoint the breakdowns that lead to the disease. The precise mechanism that led to Yamanaka’s and Thomson’s achievement last year is not yet understood, but the potential of that achievement is; it is a potential that could be unlimited.” – Ian Wilmut, Time, April 08
Click here for a graphic representation of Stem Cells.
Important Research Papers
- April 2008: Human parthenogenetic blastocysts derived from noninseminated cryopreserved human oocytes (pdf)
- November 19, 2007: Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors (pdf)
- November 3, 2007: Patient-Specific Stem Cell Lines Derived from Human Parthenogenetic Blastocysts (pdf)
- Parthenogenetic Stem Cells in NonHuman Primates (pdf)
- Nov 26, 2001: Somatic Cell Nuclear Transfer in Humans: Pronuclear and Early Embryonic Development (pdf)
Stem Cell Gold Rush
California’s landmark stem cell research program made headlines nationally, but what’s the latest story behind the science? QUEST investigates the potential for medical breakthroughs in the next decade and how the Bay Area is leading the way.
Stem Cell Gold Rush Educator Guide
Use this television story and educator guide to help your students learn exactly what stem cells are, their potential to help scientific research, and why their use has been controversial. 110b_stemcellgoldrush.pdf (406 KB)




